Past research
The contents of this page is indicative and may not be up-to-date.
Publication (review article):
Mechanical requirements for membrane fission (2009)
Dynamin
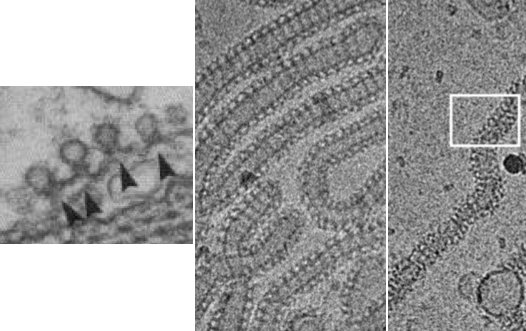
Electron micrographs of dynamin helices in cells and in vitro. [Koenig and Ikeda 1989, Danino et al. 2004] |
IntroductionDynamin is a protein implicated in breaking cell membrane tubules in eukaryotic cells, in particular during clathrin-coated endocytosis. To do so, it forms a helical polymer around membrane tubes and, upon addition of GTP, it changes conformation and breaks them according to an unknown mechanism. As described below, we study three aspects of dynamin polymers: nucleation, growth and change of conformation. Collaborators:Patricia Bassereau, Gerbrand Koster, Sandrine Morlot, Aurélien Roux, Jean-François Joanny, Jacques Prost |
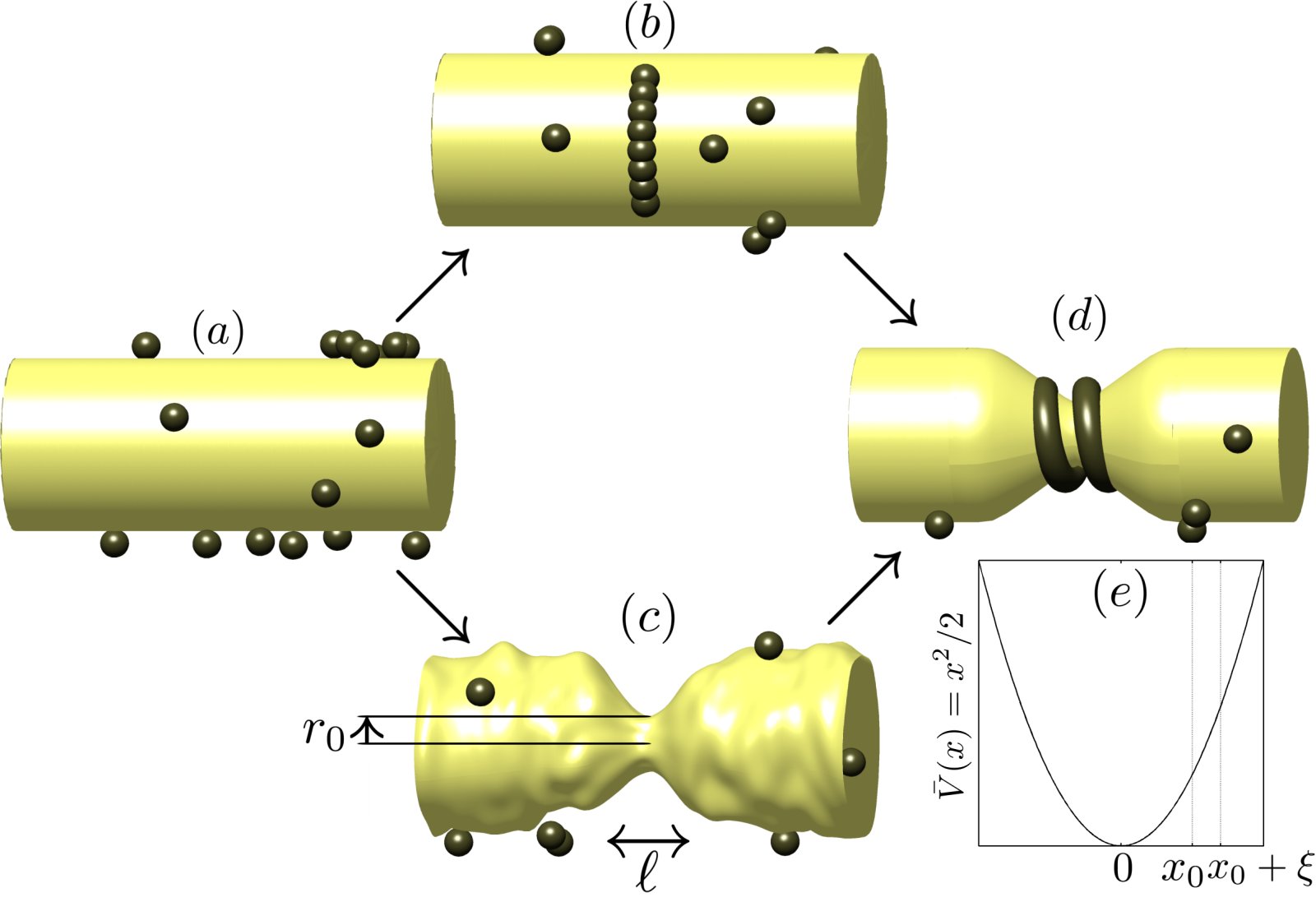
Illustration of the two extreme paths to dynamin nucleation we consider. |
NucleationWe first approach dynamin nucleation from a thermodynamic point of view. By comparing the energies associated with dynamin polymerization and membrane deformation, we predict a critical radius for dynamin nucleation, which is confirmed experimentally. We then investigate the nucleation rate of dynamin helices around a tubular membrane template of fixed radius. In order to guide experimental investigations, we consider two extreme cases and predict scaling laws for the nucleation rate as a function of membrane tension and dynamin concentration. The first hypothesis represents the case where the membrane is relatively stiff and remains undeformed while short, underbent dynamin oligomers form around it. The second, opposite hypothesis assumes that dynamin is relatively stiff and that the membrane has to thermally fluctuate down to a radius ~ 10 nm for a helical dynamin seed to form. Publication: |
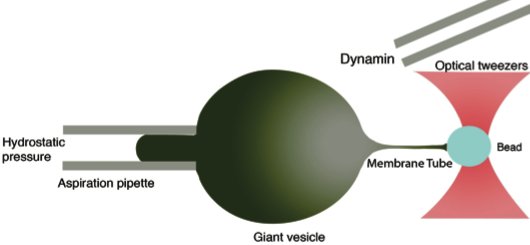
Schematic of the experimental setup used by Sandrine Morlot and Aurélien Roux in Institut Curie. Optical tweezers pull a membrane tubule from a tension-controlled vesicle of known bending modulus. Then dynamin is injected in the experimental chamber and its growth dynamics on the tubule is observed in confocal fluorescence microscopy. |
GrowthOnce dynamin polymers are nucleated, they grow on until they cover the whole membrane tubule. Experiments done in Institut Curie have uncovered surprising features of this growth process, which we investigate:
|
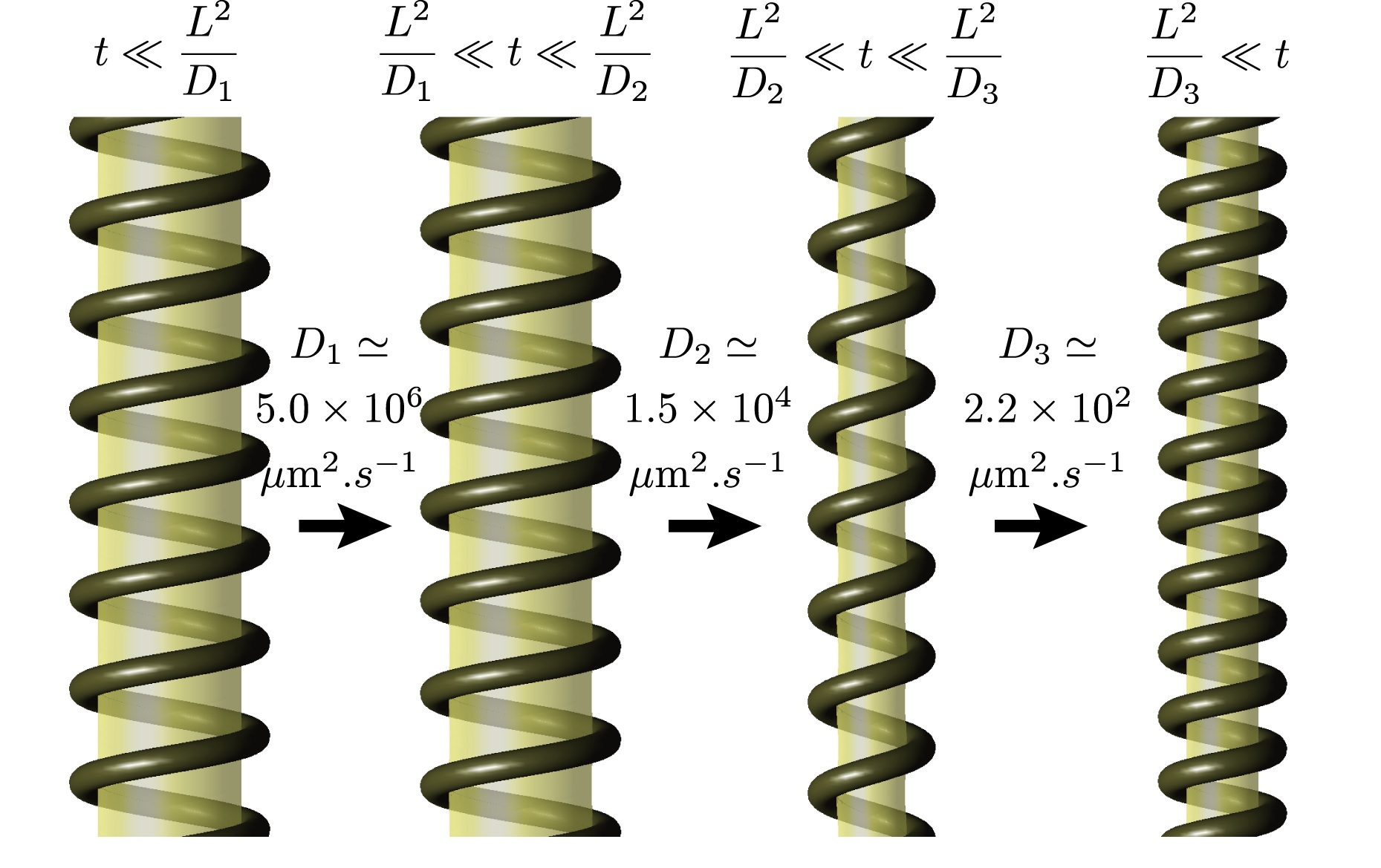
Given the fact that it assumes only the symmetries of the system, our theory involves unknown transport coefficients which have to be determined experimentally. Once this is done, one can predict the dynamics of the change of conformation of the polymer over experimentally inaccessible timescales, as shown here. |
Conformational changeLittle is known about the mechanism of conformational change of dynamin, except for the helical geometry of the polymer and the fact that it consumes energy in the form of GTP. Optimally exploiting these minimal characteristics as well as constraints imposed by thermodynamics, we develop a generalized hydrodynamics theory of dynamin-membrane tubes, which yields the following results (among others):
Publications:
Predicting dynamin's conformational change (2008) |
Stereocilia Shape
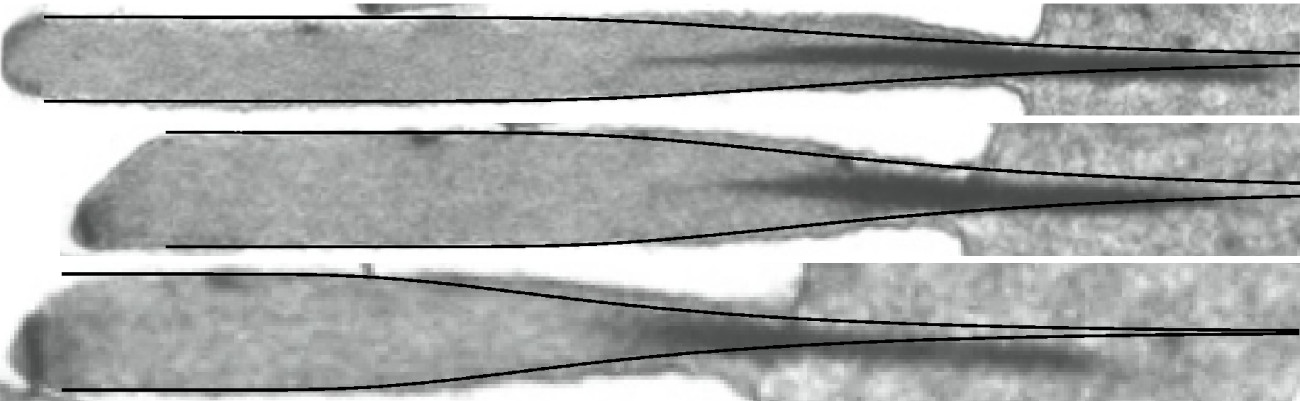
Comparison between our predictions (solid black lines) and actual stereocilia shapes |
Stereocilia are rod-like structures found in the inner ear of mammals, birds and reptiles that oscillate with sound. They are mostly made of an hexagonal array of parallel, cross-linked actin filaments. Their resonance frequency, which is closely linked to their shape, plays a crucial role in the conversion of the sound stimulus into nerve influx. Introducing the role of actin cross-linkers in a previous model [Prost 2007], we investigate the role of stochastic actin depolymerization in the morphogenesis of stereocilia. Neglecting the cross-linkers reattachement rate, we find a robust shape in good agreement with experimentally observed profiles. Allowing for cross-linkers reattachement, we observe that the height-height correlation length increases and diverges at the critical point where a stationnary stereocilium profile ceases to exist. We are currently looking into the description of this transition and developing a mean-field theory for the description of stereocilia profiles. Collaborators:Jean-François Joanny, Jacques Prost Publication: |
Active Fluctuations of red blood cells and "artificial cells"
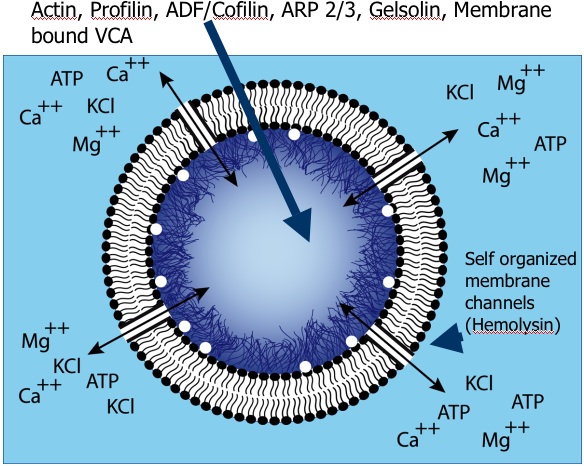
Composition of the "artificial cells" used in the Sykes group's experiments. [Pontani et al. 2009] |
The equilibrium fluctuations of cell-membrane-like lipid bilayers are rather well understood from a physical point of view. Living cells, however, typically operate well out-of-equilibrium. We provide theoretical support for the interpretation of experiments led in the group of Cécile Sykes. These include measurements of the power spectrum of the fluctuations of red blood cells (including violations of the fluctuation-dissipation theorem) and of vesicles with a polymerizing actin cortex under the membrane (and potentially myosins as well). Collaborators:Timo Betz, Cécile Sykes, Jean-François Joanny, Jacques Prost Publication:ATP-dependent mechanics of red blood cells (2009) |
ESCRT-III buckling
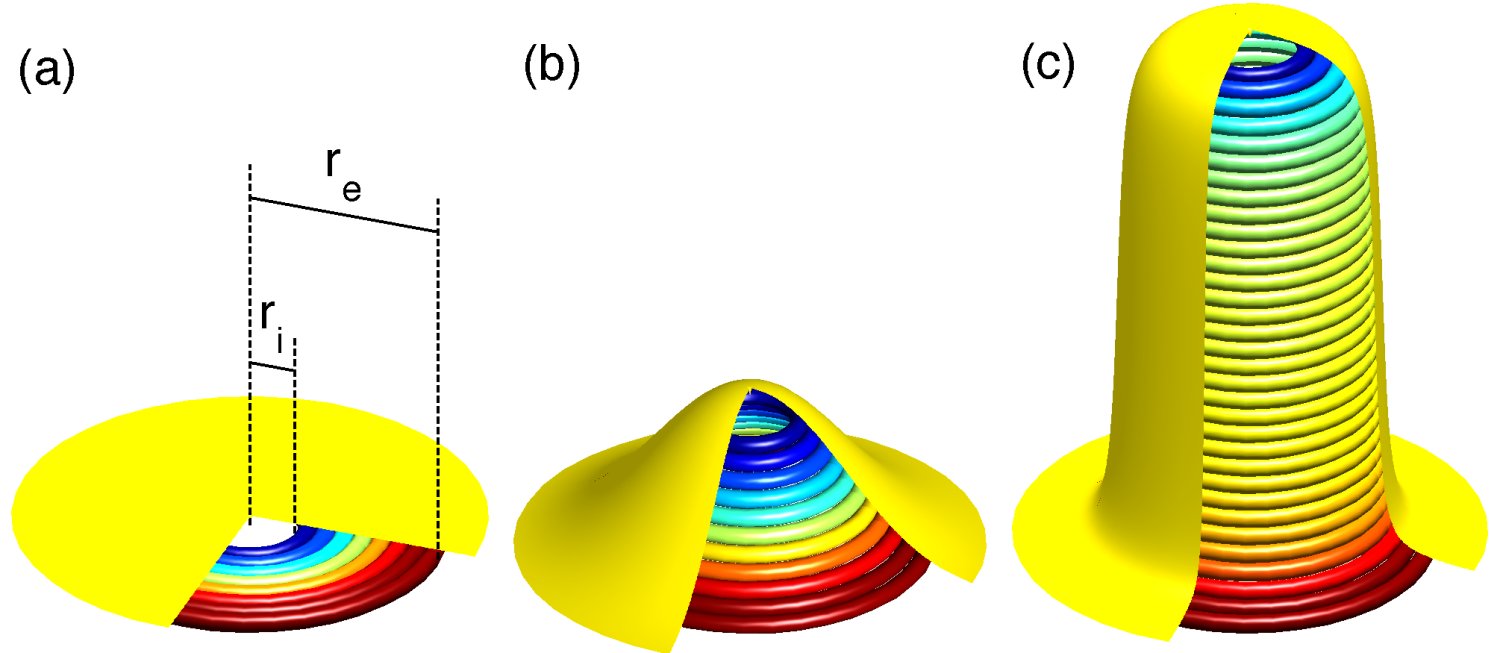
Proposed mechanism for ESCRT-III-induced membrane deformation. The stress in overbent (blue) and underbent (red) hSnf-7 filaments relaxes as the flat array buckles and forms a long tube, which is observed experimentally. This has the additional advantage of allowing more hSnf-7 filaments to bind to the membrane. |
ESCRT-III is a protein complex implicated in the formation of membrane evaginations in eukaryotic cells, for instance during the exocytosis of the HIV virus. When overexpressed, one of its components, hSnf-7, polymerizes into filaments that form a circular array under the plasma membrane. Then these arrays bud outwards and are cut by another protein, forming topologically independent, membrane-coated vesicles. We describe the plane-to-evaginated transition of the hSnf-7 arrays as a buckling transition due to the build-up of elastic stresses in the naturally curved hSnf-7 filaments. Collaborators:Daniel J. G. Crow, Jean-François Joanny Publication: |
Contact inhibition
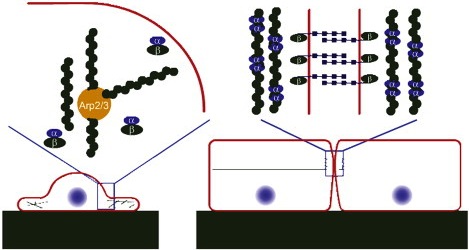
Illustration of the interplay between the cadherin-catenin system and the cytoskeleton, leading to contact inhibition |
Contact inhibition is the process by which cells switch from a motile growing state to a passive and stabilized state upon touching their neighbors. When two cells touch, an adhesion link is created between them by means of transmembrane E-cadherin proteins. Simultaneously, their actin filaments stop polymerizing in the direction perpendicular to the membrane and reorganize to create an apical belt that colocalizes with the adhesion links. We propose a reaction-diffusion model of this process that could explain previously unrelated experimental findings on the role played by the proteins E-cadherin, α-catenin, and β-catenin in the cellular phenotype and in tumorigenesis. Collaborators:Markus Basan, Timon Idema, Thomas Risler, Jean-François Joanny Publication:Proposing a molecular mechanism for contact inhibition (2010) |
Polymerization of and chemoattraction by MIP-1
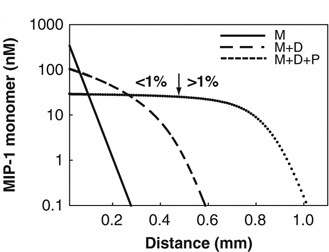
Spatial profiles of MIP-1 concentration away from an inflammation site. Oligomerization of MIP-1 protects it against degradation, allowing it to diffuse over larger distances. We propose that this process could mediate a stronger immune response to serious infections. |
The macrophage inflamatory protein-1 (MIP-1) is a chemoattracting protein crucial for targetting the immune response against infection and inflammation. Crystallographic data from the Tang lab at the University of Chicago show that MIP-1 dimers polymerize into long helical polymers. We provide a thermodynamic analysis of the polymer size distribution and use it to analyze MIP-1 X-ray scattering data. A structural study moreover suggests that polymerization protects MIP-1 from degradation. Using a reaction-diffusion model, we study the implications of this phenomenon for MIP-1 signaling. Collaborators:Min Ren, Qing Guo, Wei-Jen Tang, Aaron Dinner Publication: |
Design of nanoparticles for medical imaging and drug delivery
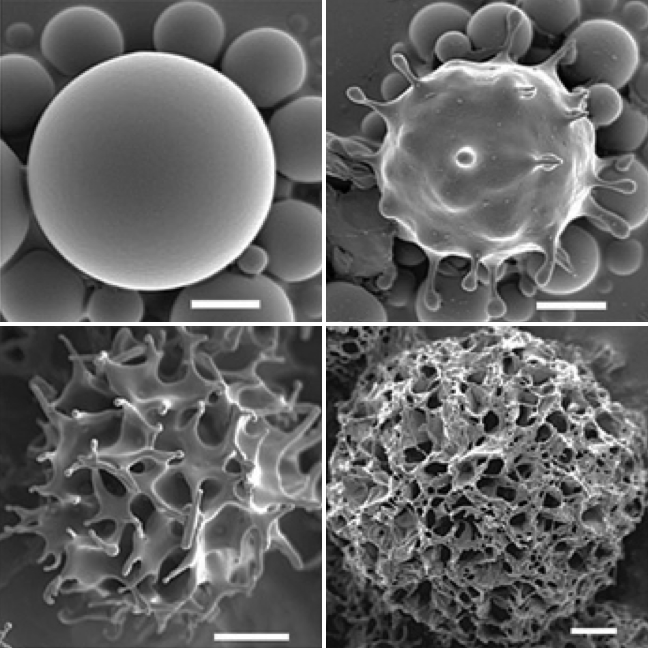
A surface instability turns the smooth nanoparticles studied in collaboration with Tsapis and Guenoun's groups into sponge-like objects. |
Nanoparticles are a promising avenue for improved cancer diagnostics and treatment. We study self-assembled nanometric capsules comprising a liquid perfluorocarbon core encased in a glassy polymer shell. These capsules can be injected intravenously for use as ultrasound or F-MRI contrast agents, as well as drug delivery devices. To optimize their function, it is desirable to passivate and/or functionalize the capsules. This poses technological challenges which we help understand and solve through theory. Additionally, we model the response of particles probed by atomic force microscopy (AFM) to characterize their mechanics and performance as ultrasound contrast agents. We also attempt to relate these characteristics to their ultrasound-induced rupture and resulting drug delivery potential. Collaborators:Nicolas Tsapis, Patrick Guenoun |